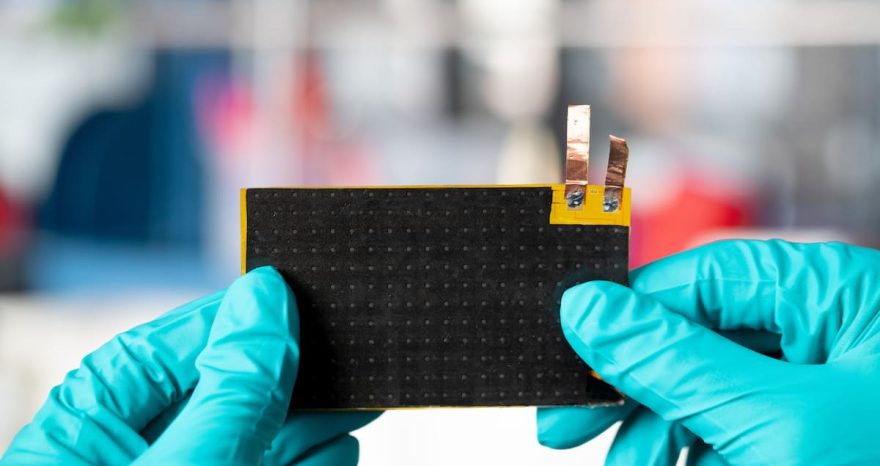
Developed a fast and sustainable method to generate hydrogen that is economically competitive with current electrolysis-based production

A new low-emission pathway to produce hydrogen from seawater
Seawater, spent coffee grounds, and discarded aluminum cans might soon become unexpected allies in clean hydrogen production. In 2024, a team of engineers at the Massachusetts Institute of Technology (MIT) introduced a fast and sustainable method to generate hydrogen gas with minimal environmental impact.
In a study published in Cell Reports Sustainability, the researchers presented the first life-cycle assessment of this process. The results? Producing hydrogen from aluminum and seawater emits just 1.45 kg of carbon dioxide per kilogram of hydrogen, far below the 11 kg associated with fossil-fuel-based methods.
But to fully grasp the potential of this technology, we need to start from the basics.
The aluminum-water reaction that unlocks hydrogen
Previous research revealed that aluminum can react with water to release hydrogen and heat, but only if the metal is mechanically activated. This activation is essential because simply dunking an old aluminum can in water won’t trigger any chemical reaction.
Why? Because aluminum naturally forms a protective oxide layer when exposed to oxygen. This layer prevents further reactions. Remove the oxide, and aluminum can interact with water to generate aluminum oxide and pure hydrogen gas.
To enable this, researchers use a process called activation. It involves weakening the metal’s structure with low-melting-point alloys, such as gallium and indium, allowing water to penetrate the oxide shield.
However, pre-treating aluminum with rare metal alloys is costly and impractical for commercial-scale production. That’s where MIT’s innovation comes in. In 2024, PhD candidate Aly Kombargi from MIT’s Department of Mechanical Engineering and his colleagues took the concept to the ocean.
Seawater contains ions that naturally attract and recover the gallium-indium alloy, enabling its reuse across multiple reaction cycles.
Caffeine compound boosts speed and efficiency
To further speed up the hydrogen production, cutting the reaction time to under 10 minutes, the team added imidazole, a molecule found in caffeine. Imidazole perforates the aluminum’s surface, allowing continuous reaction with water, while leaving the gallium-indium layer intact.
So how much hydrogen can this method produce? Other studies estimate that just 1 gram of pre-treated aluminum pellets can yield 1.3 liters of hydrogen gas in five minutes.
Evaluating the environmental and economic impact
With proof of concept in hand, the researchers analyzed whether this method could scale sustainably. They assessed the full emissions lifecycle, from aluminum acquisition and processing to the seawater reaction and fuel transportation.
Their calculations showed that the method releases only 1.45 kg of CO2-equivalent per kg of hydrogen, on par with clean alternatives like electrolysis.
Cost-wise, hydrogen produced this way would run about $9 per kilogram, comparable to green hydrogen. But the price could fall even further. The reaction also produces boehmite, a valuable mineral used in semiconductors, electronics, and industrial products. Recovering and selling boehmite could help offset initial production costs.
Reference:
Read the full study Life-cycle assessment and cost analysis of hydrogen production via aluminum-seawater reactions in Cell Reports Sustainability (2025).