A new chemical battery system uses gaseous hydrogen as the cathode and lithium metal as the anode
The University of Science and Technology of China is pioneering the use of catalytic gaseous hydrogen for high-performance energy storage applications. This breakthrough comes from a new lithium-hydrogen battery developed by chemist Zaichun Liu and colleagues, who have created a prototype with remarkable electrochemical properties.
What are hydrogen batteries?
The idea of using hydrogen in rechargeable batteries has gained significant attention in recent years. Since 2020, research on so-called protonic batteries has expanded, exploring the use of protons (hydrogen ions) instead of lithium ions as charge carriers.
At the same time, metal-hydrogen batteries have emerged as potential alternatives to metal-air batteries. These systems typically use a metal hydride (a material that absorbs hydrogen) as the anode and a metal oxide or hydroxide as the cathode. A well-known example is nickel-metal hydride (NiMH) batteries.
However, the Chinese research team has demonstrated a third approach. Seeking to significantly improve energy density and operating voltage, they employed gaseous hydrogen as the cathode and lithium metal as the anode.
The new lithium-hydrogen batteries: with and without an anode
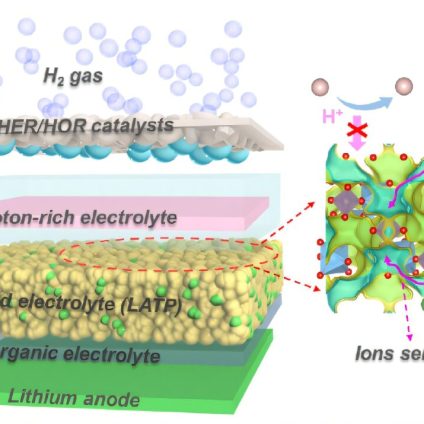
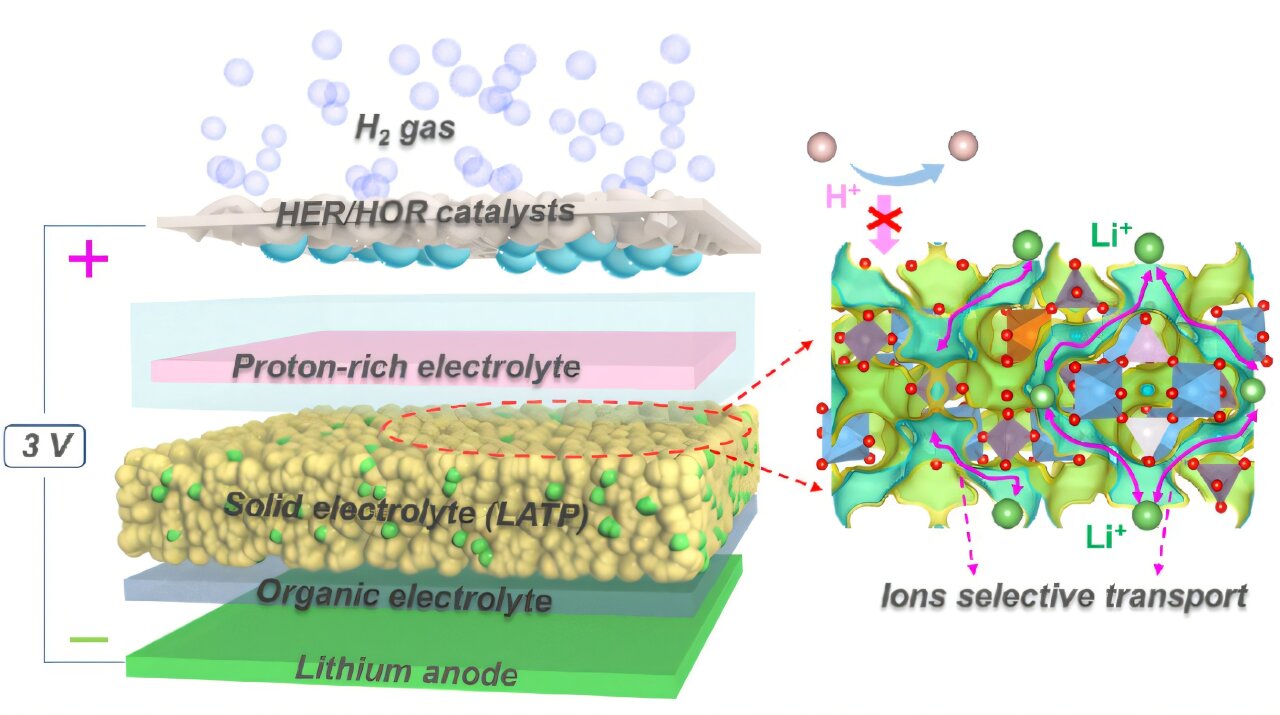
The team designed a prototype lithium-hydrogen (Li-H) battery system incorporating, in sequence: a lithium metal anode, an organic liquid electrolyte, a solid electrolyte layer (ceramic LATP material), a proton-rich electrolyte, and a gas diffusion layer coated with platinum serving as the hydrogen cathode. According to the researchers, this configuration ensures efficient lithium-ion transport while minimizing unwanted chemical interactions.
Test results show that the new lithium-hydrogen battery achieves an impressive theoretical energy density of 2,825 Wh/kg while maintaining a stable voltage of approximately 3V. Additionally, the unit demonstrated a round-trip efficiency (the percentage of stored energy that can be effectively used) of 99.7%, indicating minimal energy loss during charge and discharge cycles. The battery also maintains long-term stability.
To further enhance cost efficiency, safety, and manufacturing simplicity, the researchers developed an anode-free rechargeable Li-H battery, eliminating the need for pre-installed lithium metal. Instead, during charging, lithium is deposited from lithium salts (LiH2PO4 and LiOH) within the electrolyte.
“This version retains the advantages of the standard lithium-hydrogen battery while introducing additional benefits. It enables efficient lithium plating and removal with a Coulombic efficiency of 98.5%. Moreover, it remains stable even at low hydrogen concentrations, reducing dependence on high-pressure H₂ storage.”
The research has been published in Angewandte Chemie International Edition.