A new artificial photosynthesis system has been developed for direct solar-to-hydrogen conversion, meeting high efficiency, long-term stability, and scalability requirements.
From sunlight to hydrogen with 11.2% efficiency
Photoelectrochemical hydrogen production has long been little more than a daydream. Not because it was technically impossible to split water into hydrogen gas using sunlight, but because the process has historically been inefficient and difficult to scale.
Now, a team of chemical engineers at the Ulsan National Institute of Science and Technology (UNIST) in South Korea has made a breakthrough. The researchers have developed an artificial photosynthesis system capable of producing solar hydrogen with a conversion efficiency of 11.2%, while also achieving high operational stability.
“This result goes beyond lab-scale demonstrations, reaching a module-level efficiency above 10%, a critical benchmark for real-world applications,” explained professor Jae Sung Lee, who led the project.
Technologies behind photoelectrochemical hydrogen production
Producing hydrogen directly from sunlight requires specialized devices that can use solar energy to split water molecules. The two most common approaches are photovoltaic-electrochemical systems and photoelectrochemical systems.
Photovoltaic-electrochemical setups combine separate photovoltaic and electrochemical cells, requiring significant space and high upfront costs.
Photoelectrochemical systems, on the other hand, integrate both components into a single device, creating a more compact solution. However, these systems often rely on external wiring, which increases resistivity and reduces overall efficiency.
A promising alternative in this category is the artificial leaf. These devices mimic natural photosynthesis by integrating light-absorbing semiconductors and electrochemical cells into a single structure.
The challenges of artificial photosynthesis
Artificial leaves show great promise on paper but have struggled to move beyond the lab. The main challenge for artificial photosynthesis systems is achieving high conversion efficiency while also being durable and scalable for commercial applications. After more than a decade of research, no artificial leaf had succeeded in meeting all three of these requirements, until now.
Researchers have often faced a trade-off between stable but inefficient semiconductors and efficient ones prone to rapid degradation. This has made it difficult to develop large-area photoelectrodes suitable for commercial-scale devices.
UNIST’s perovskite-based artificial leaf
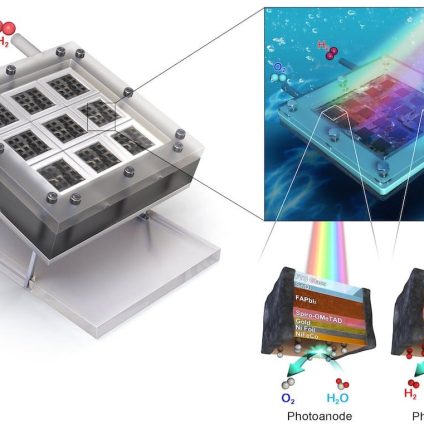
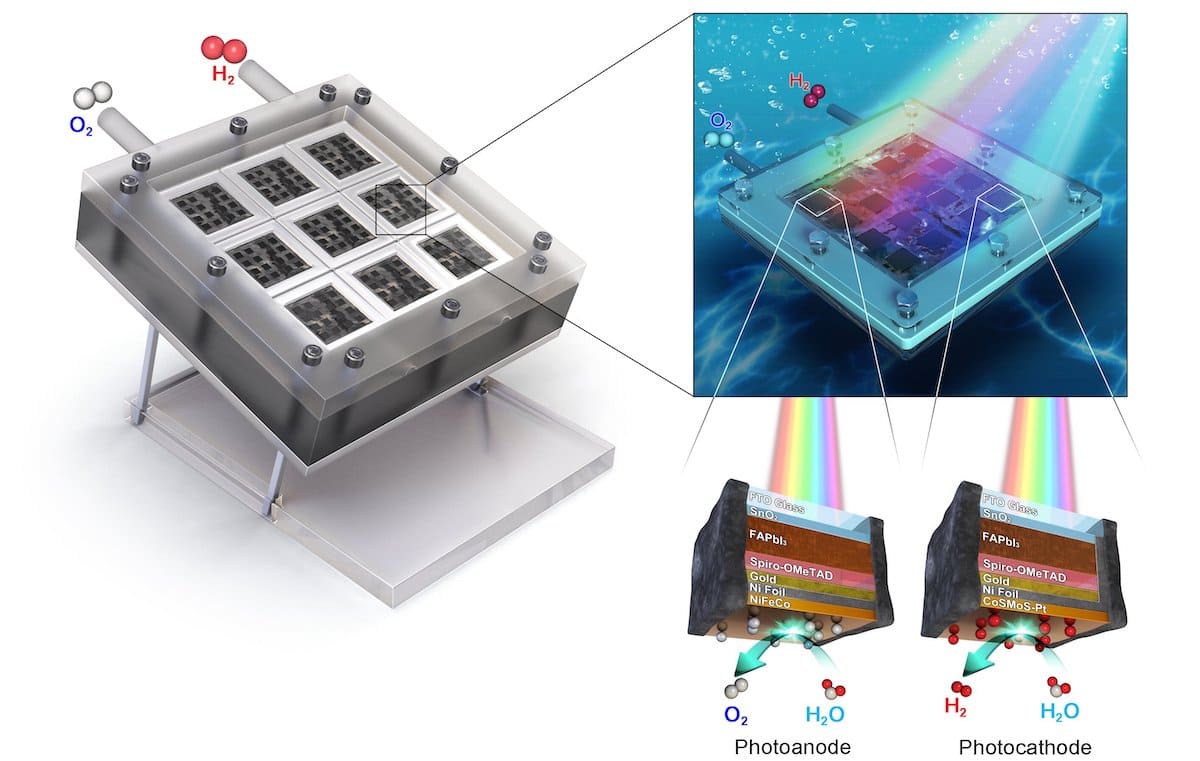
The UNIST team found a way to solve all three issues at once. They developed new 1 cm² photoelectrodes using formamidinium lead triiodide (FAPbI₃), a perovskite doped with chlorine, as the light absorber, and UV-resistant tin oxide as the charge transport layer. The electrodes were encapsulated using nickel foil and resin applied via electrocatalysis.
These units were assembled into a mini-module artificial leaf containing eight photoanodes and eight photocathodes, covering a surface area of 16 cm². The result is a scalable system for photoelectrochemical hydrogen production.
The outcome? The module achieved a solar-to-hydrogen conversion efficiency of 11.2%, surpassing the 10% threshold considered essential for commercial viability. Even more impressively, the device operated continuously for 140 hours while maintaining 99% of its initial performance.
The study, Scalable and durable module-sized artificial leaf with a solar-to-hydrogen efficiency over 10%, was published in Nature Communications.